what is the valence electrons of selenium|How to Find the Valence Electrons for Selenium (Se) : Manila There are two ways to find the number of valence electrons in Selenium (Se). The first is to use the Periodic Table to figure out how many electrons Selenium has in its valence shell.. Best Vermont Online Sportsbooks for 2024. To form a proper ranking of Vermont sports betting sites, we performed extensive research into all available operators.We looked at all the crucial factors that gamblers are interested in, such as markets, bonuses, features, bet types, mobile support, and more.
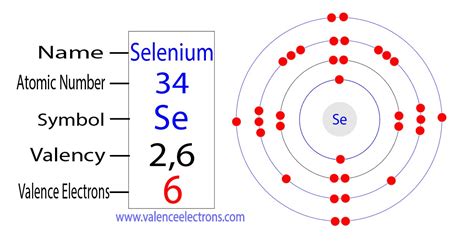
what is the valence electrons of selenium,The 3rd element in group-16 is selenium. The valence electrons are the total number of electrons in the last orbit(shell). The total number of electrons in the last shell after the electron configuration of seleniumis called the valence electrons of selenium. The valence electrons . Tingnan ang higit pa
The valence electron has to be determined by following a few steps. The electron configuration is one of them. It is not possible to . Tingnan ang higit paThe elements that have 5, 6, or 7 electrons in the last shell receive the electrons in the last shell during bond formation. . Tingnan ang higit paThe ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). There are some rules for diagnosing valency. The . Tingnan ang higit pa There are two ways to find the number of valence electrons in Selenium (Se). The first is to use the Periodic Table to figure out how many electrons Selenium has in its valence shell..
Valences of the Elements Chemistry Table. You may assume that the .
In the chemistry branch of science, Selenium has recognition as the chemical element. It has the symbol of Se and the atomic number 34. Flerovium Valence Electrons. Helium Valence Electrons. . A selenium atom has six valence electrons. Do you think it will lose six electrons or gain two electrons to obtain an octet in its outermost electron shell? Write .
Atomic and physical properties of selenium. View Large. In nature, selenium is found in ore minerals, partially substituting sulfur. Selenium can exist as . Selenium has 6 valence electrons because there are 6 electrons present in the outermost shell of the Selenium (Se) atom. Now let’s see how you can easily find . Selenium has 6 valence electrons. Methods. We can write the valence electrons of selenium using two different methods: #1 Using periodic table. #2 Using electron configuration. Let’s break down each . Valence electrons are the electrons which are located in the outermost shell of the atom or molecule. Selenium has 6 electrons in its outermost shell i.e. 2 electrons in s orbit and 4 electrons in the p .what is the valence electrons of selenium Selenium consists of 34 electrons distribution in its 4 orbits. So electronic configuration of selenium define as: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 3d10 4p 4. Or. The electronic configuration can also be .The atomic number of selenium is 34, which means it has 34 electrons. Now it is possible to find the orbital notation of selenium very easily through electron configuration. That is, the orbital notation of selenium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 4.Protons and Neutrons in Selenium. Selenium is a chemical element with atomic number 34 which means there are 34 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the .
Selenium: Element Number 34. Selenium is a nonmetal mineral essential to human metabolism; it can be found in certain foods, soil and water. To identify an element's valence or outermost electrons, you can refer to its group number on the periodic table.

Method 1: From the Periodic Table. To find out the valence electrons of Selenium, you have to see the position of selenium in the periodic table. More specifically, you have to see the group wise position of Selenium element in the periodic table. From the above image, you can see that the Selenium (Se) is present in the group 16 of .Selenium is a member of the sulfur family with elements including tellurium and polonium. This family has six electrons in the outermost shell. Selenium specifically has an electron configuration of 2-8-18-6. The six electrons in the outermost shell allow selenium to have a variety of valence numbers. Selenium compounds have been found that . The selenium valence electronic configuration ([Ar] 3d 10 4s 2 4p 4) is equivalent to that of sulfur ([Ne] . Electron capture. Radioactive decay in which an electron is absorbed by the nucleus that simultaneously emits an electron neutrino. Observed in atom nuclei with an abundance of protons but not enough energy to .Orbital diagram. Selenium electron configuration. ← Electronic configurations of elements. Se (Selenium) is an element with position number 34 in the periodic table. Located in the IV period. Melting point: 217 ℃. Density: 4.82 g/cm 3 . Electronic configuration of the Selenium atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 .
If you knew the number of valence electrons in a nonmetal atom, how would you determine the valence of the element . (they all have eight valence electrons so they are happy as is) Selenium has six valence electrons. What is the valence of selenium. Two. The number of valence electrons plus the valence equals the . Sum. About us. About .Selenium is a classified nonmetal and its symbol is ‘Se’. Selenium is the 34th element of the periodic table so its atomic number is 34. The atomic number of an element is equal to the number of protons and electrons in that element. Therefore, a selenium atom has thirty-four protons and thirty-four electrons.
1.3: Valence electrons and open valences. A valence electron is an electron that is associated with an atom, and that can participate in the formation of a chemical bond; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. The presence of valence electrons can determine the element's . David Jin (UCD) Chemistry of Selenium (Z=34) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Element number 34, selenium, was discovered by Swedish chemist Jons Jacob Berzelius in 1817. Selenium is a non-metal and can be compared chemically to its other non-metal . Valence electrons given by Selenium (Se) atom = 6 Valence electrons given by each Sulfur (S) atom = 6 So, total number of Valence electrons in SeS2 molecule = 6 + 6(2) = 18. Step #2: Select the center atom. While selecting the atom, always put the least electronegative atom at the center. .
valence electron, any of the fundamental negatively charged particles in the outermost region of atoms that enters into the formation of chemical bonds.Whatever the type of chemical bond (ionic, covalent, metallic) between atoms, changes in the atomic structure are restricted to the outermost, or valence, electrons.They are more weakly . Step-1: First, find the atomic number of selenium from periodic table. From periodic table ,we see that the atomic number of selenium is 34. Step-2: We know that the atomic number of selenium is 34.So selenium has 34 protons and 34 electrons as the charge of electrons and protons are equal but opposite in nature.The charge of proton .Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..
sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group .How to Find the Valence Electrons for Selenium (Se) sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group .

Total electron pairs = total valence electrons ÷ 2. So the total electron pairs = 18 ÷ 2 = 9. Third, determine the central atom; We have to place the least electronegative atom at the center. Since selenium is less electronegative than sulfur, assume that the central atom is selenium.
what is the valence electrons of selenium|How to Find the Valence Electrons for Selenium (Se)
PH0 · Valences of the Elements Chemistry Table
PH1 · Selenium valence electrons
PH2 · Selenium Valence Electrons (And How to Find them?)
PH3 · Selenium Valence Electrons
PH4 · Selenium Electron Configuration (Se) with Orbital Diagram
PH5 · Selenium Electron Configuration (Se) with Orbital
PH6 · How to Find the Valence Electrons for Selenium (Se)?
PH7 · How to Find the Valence Electrons for Selenium (Se)
PH8 · Electron Configuration for Selenium (Se, Se2
PH9 · CHAPTER 1: The Chemistry of Selenium
PH10 · 3.1: Valence Electrons